Vaccines: Definition, Step by step process and Types of vaccine
Vaccines are a crucial aspect of modern medicine that has revolutionized healthcare by protecting people from deadly infectious diseases. Vaccines work by stimulating the body’s immune system to recognize and fight specific pathogens, which can lead to immunity against the disease. The process of creating and administering vaccines involves a step-by-step process that can vary depending on the type of vaccine. There are several types of vaccines available, including live attenuated, inactivated, subunit, conjugate, and mRNA vaccines, each with its unique mechanism of action and benefits. In this article, we will explore the definition of vaccines, the step-by-step process of vaccine development, and the different types of vaccines.
Vaccines –An introduction
A biological remedy that increases resistance to a particular disease is vaccination. A substance resembling a disease-causing germ is regularly used in vaccinations; it is frequently made from a bacteria’s less-powerful or dead forms, its toxins, or one of its surface proteins.
The material causes the immune system to become activated, allowing it to recognize and destroy the substance as a threat, as well as any subsequent microorganisms it may come into touch with that, are associated with it. In China, where smallpox was first eradicated one hundred years ago, the term “injection” was originally used to describe the immunization method. A bacterial or viral antigen, such as a bacterium or virus, is typically used in manufacturing. This antigen may be dead or it may be alive but attenuated. The disease-causing organism is cultured in specialized lab settings that induce it to lose its virulence or disease-causing qualities to create a live attenuated vaccine. A bacterial or viral antigen, such as a bacterium or virus, is typically used in manufacturing. This antigen may be dead or it may be alive but attenuated. The disease-causing organism is cultured in specialized lab settings that induce it to lose its virulence or disease-causing qualities to create a live attenuated vaccine.
The measles vaccine, BCG vaccine, and oral polio vaccine are three examples of live attenuated vaccinations.
Recently approved vaccinations for COVID-19 include the m-RNA vaccine.
Heat or the passage of the virus via a non-native host, such as embryonated eggs or tissue culture cells, can attenuate the virus. Attenuation was only accomplished in primary monkey kidney cells with high inoculum and quick passage to create the Sabin polio vaccine. To create inactivated vaccinations, the disease-causing bacteria must be destroyed using chemicals or heat.
• Nowadays, gene methods are used to manufacture vaccines, i.e. instead of using a virus or bacterium, A single gene (usually a surface glycoprotein of the virus) can be expressed in a foreign host by Cloning (Expression vectors are employed to produce vast quantities of antigen for vaccines). Bacteria (Escherichia coli, yeast, and baculovirus) are the most common expression vectors.
Types of vaccine| vaccines and their types:
- Inactivated vaccine
- Live vaccines
- Viral vectors vaccines
- Toxoid vaccine
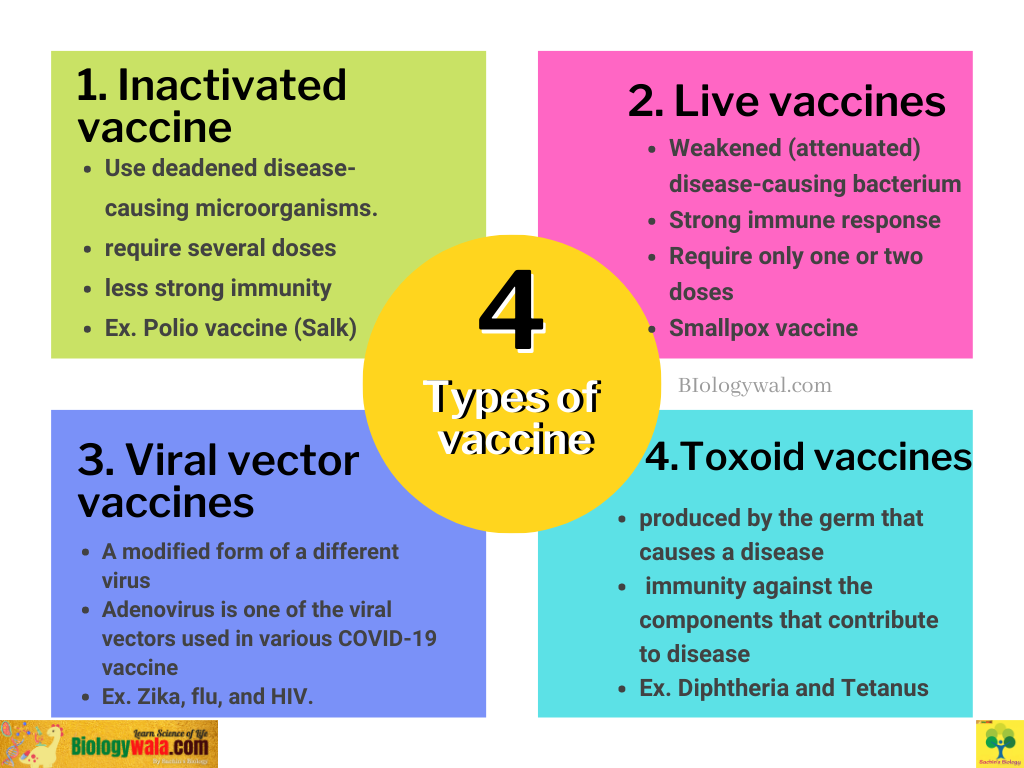
1. Inactivated vaccine
Inactivated vaccines use deadened disease-causing microorganisms. Inactivated vaccines typically will not provide as strong of immunity (protection) as live vaccines. Thus, several doses (booster shots) throughout time may be necessary to maintain your immunity against illnesses.
2. Live vaccines
Live vaccines make use of a disease-causing bacterium that has been weakened (or attenuated). Because they closely resemble the natural infection that they help prevent, these vaccinations generate a strong immune response. For lifetime protection against a germ and the disease it causes, most live vaccinations only need one or two doses. But live vaccines have significant drawbacks as well.
3. Viral vectors vaccines
Viral vector vaccines have been the subject of extensive research. Viral vector technology has been used in some vaccinations lately used to combat Ebola outbreaks, and several studies have concentrated on viral vector vaccines against other infectious diseases like Zika, flu, and HIV. This method was also employed by scientists to create COVID-19 vaccinations.
Viral vector vaccines give protection by using a modified form of a different virus as a vector. The influenza virus, the vesicular stomatitis virus (VSV), the measles virus, and the adenovirus that causes the common cold have all been utilised as vectors. One of the viral vectors employed in various COVID-19 vaccines currently undergoing clinical testing is an adenovirus. Vaccines against viral vectors are used to guard against COVID-19.
4. Toxoid vaccine
A toxin (harmful substance) produced by the germ that causes a disease is used in toxoid vaccinations. Instead of the germ itself, they develop immunity against the components that contribute to disease. In other words, the immune response is focused on the poison rather than the entire germ. These types of vaccines are cast-off to defend against Diphtheria, Tetanus.

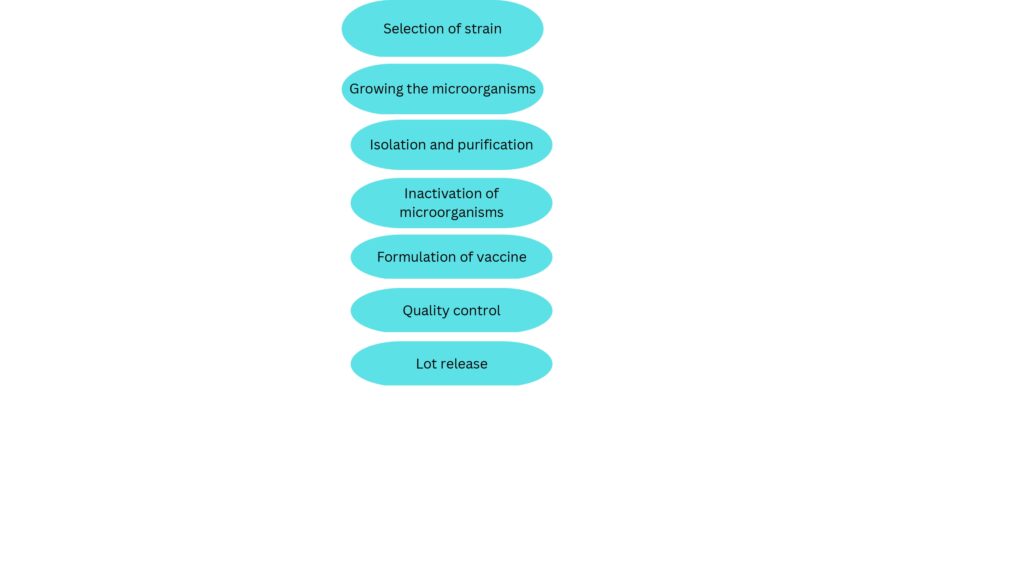
STEPS IN VACCINE PRODUCTION | How are vaccines made step by step?
- SELECTING THE STRAINS FOR VACCINE PRODUCTION
- GROWING THE MICROORGANISMS
- ISOLATION & PURIFICATION OF MICROORGANISMS
- INACTIVATION OF ORGANISM
- FORMULATION OF VACCINE
- QUALITY CONTROL
- LOT RELEASE
1. SELECTING THE STRAINS FOR VACCINE PRODUCTION
The Seed (Strain) – Production starts with trace levels of a particular virus (seed). – Bacteria or viruses used in manufacturing must come from a seed lot system.
• For each Seed Lot, a record of the place of origin, the passage history (including the purification and characterization processes), and the storage circumstances should be kept.
• The virus must be devoid of contaminants, such as other viruses that are identical to it or even other strains of the same virus.
• To prevent the virus from getting either stronger or weaker than desired, the seed must be stored under “optimal” conditions, typically frozen.
• Put in a small glass or plastic container.
Selecting the seed (Strain)
– The effectiveness of the resulting vaccination and any side effects have an impact on the choice of the seed, among other things.
– The bacterial strain or cell line should, if at all possible, come from a reputable culture collection with a provenance that has been established and documented.
– Conversely, if the chosen vaccination strain is an “in-house” clinical isolate, a full history of the strain, including information on its isolation, identification, and upkeep for product registration, will need to be compiled.
2. GROWING THE MICROORGANISMS
Growing bacteria
Methods used are: –
-BATCH CULTURE
• the microbe is cultured in a closed vessel
• characteristically, in a test tube or flask
-CONTINUOUS CULTURE
• The microorganism is cultured in a chemostat, where the fresh medium is continuously introduced and used media is continuously withdrawn.
• It is carried out in a chemostat.
Growing viruses
CELL (TISSUE) CULTURES – Cultured cells grow in sheets that enable viral replication and allow the study of the cytopathic effect.
• BIRD EMBRYOS – An appropriate system for injecting a virus is an egg that is incubating.
• LIVE ANIMAL INOCULATION – rarely employed
• TRANSGENIC ANIMALS
3. Isolation & Purification of microorganisms
• The removal of components whose qualities significantly differ from those of the desired product is known as product isolation.
• Purification carefully separates and holds onto the intended product with the maximum purity by its predetermined specification.
(Remove undesirable substances)
• A fermentation process followed by purification is the foundation of the most popular vaccine production technique.
- CENTRIFUGATION
- FILTRATION
- CHROMATOGRAPHY
Centrifugation
By applying centrifugal force as a driving force, solid particles are sedimented and separated from a liquid during centrifugation. Pathogenic virus antigens and other substances needed in the creation of vaccines are separated and purified using centrifugation. The removal of dead cells, cell debris, etc. is another function of centrifugation. The creation of vaccines against influenza, rabies, hepatitis B, and Japanese encephalitis are a few examples. Types of centrifugations:
Differential Centrifugation
Density gradient Centrifugation
Differential Centrifugation
This method, which involves varying speeds and timings, is used to separate cell organelles. The resulting pellet and supernatant are then treated to various speeds at various periods, the supernatant is then removed, and the procedure is repeated. Little particles are left in the supernatant when fractions separate at low rates. Depending on their size, shape, and density, cells, organelles, and macromolecules can be separated using the density gradient centrifugation technique.
Density gradient Centrifugation
Depending on their size, shape, and density, cells, organelles, and macromolecules can be separated using the density gradient centrifugation technique.
a. Rate zonal centrifugation-Using rate-zonal centrifugation, molecules are separated according to their size, shape, and density.
b. Isopycnic centrifugation, a method for classifying molecules according to density. (The definition of “isopycnic” is “equal density”).
- Filtration
separation of particles from a liquid by forcing the solution through a filter while applying pressure to the fluid.
Two categories are used to classify filtration:
1)Dead-end filtration:
Materials accumulate on the filter’s surface while all flows are routed through the membrane. (Flow parallel to membrane surface)
As these particles accumulate, flow through the filter rapidly declines until it eventually stops entirely. (Results in filter cake accumulation on the membrane))
2)Tangential filtration (CROSS FLOW TECHNOLOGY):
CFF (cross-flow filtration) involves the tangential flow of culture fluid in a direction parallel to the filter membrane. Recirculating fluid over the membrane reduces the number of virus particles that can build up there, and it also makes it easier for those particles to concentrate in the fluid that has been retained. utilized primarily for purifying inactive Arboviral antigens.
Chromatography
A method of physical separation techniques that separates mixtures due to variations in the sample components’ distribution coefficients between two phases, one stationary and the other mobile phase.
Column Chromatography, Ion exchange chromatography, Affinity chromatography
4. Inactivation of microorganisms
KILLED/INACTIVATED VACCINE:
VIRUS INACTIVATION:
Viral particles can either be lipid-coated (enveloped) or not. Inactivating a virus requires destroying its capacity to infect cells without actually getting rid of it.
One of the two following mechanisms is used to inactivate viruses:
By eliminating the virus’ capacity to interact with or infect cells by targeting the viral envelope or capsid.
By interfering with the RNA or DNA of the virus and halting replication.
- Inactivation of solvent/detergent (S/D)
- Pasteurization
- Acidic pH inactivation (Low pH Treatment)
- Ultraviolet (UV) inactivation
Solvent/detergent (S/D) inactivation
effective with viruses with lipid coatings. The lipid coat is disrupted by the detergents used in this approach, which prevents reproduction and makes the coat defective. The majority of enveloped viruses are killed by exposure to these detergents because they cannot survive without their lipid coating. Some viruses may still exist, but because they are unable to procreate, they are no longer contagious. Triton-X 100 is the common detergent that is employed.
Pasteurization
Efficient against lipid- and non-lipid-coated viruses. No matter if the virus has an envelope or not, pasteurization requires raising the temperature of the solution to a level that will sufficiently denature the virus. The virus cannot withstand such high temperatures (at 60 °C for 10 hours).
Acidic pH inactivation (Low pH Treatment) –Most effective with lipid-coated viruses.
Acidic environments render viruses inactive.
It usually takes 6 hours to 21 days for incubation to take place at a pH of 4.
Ultraviolet (UV) inactivation
As virus particles are small and UV rays may penetrate genetic material, dimerizing nucleic acids, they can be utilized to inactivate viruses. The virus particles are unable to replicate their genetic material once the DNA has dimerized.
5. Formulation of vaccine
Salts, preservatives, stabilizers, and buffer components may be used in the production of vaccines. Whether added, stored, or used, these additions shouldn’t hurt the vaccine’s active ingredients. Antibiotics, phenoxyethanol, phenol, and thimerosal are some of the stabilizers and preservatives utilized. Aluminium sulphate, potassium aluminium sulphate, and aluminium hydroxide are frequently used adjuvants in vaccines.
• The stabilizers monosodium glutamate and 2-phenoxyethanol, which assist certain vaccines maintain their integrity when exposed to heat, light, acidity, or humidity. • By doing this, the gastrointestinal tract’s lytic enzymes and low pH may not degrade them.
6. Quality control
AN IMPROVEMENT IN VIRUS TESTS
When an organism is used in a live vaccination, there is a chance that it will shed from the host and spread to other animals it comes into contact with, potentially infecting them with the disease if any virulence remains or returns. Passage studies should be used to determine the pathogenicity of all live vaccinations.
ESTIMATION OF ENVIRONMENTAL RISK
To assess the risk of a live vaccine to the environment while taking into account human health, it is necessary to examine each live vaccine’s capacity to shed, spread to encounter target and non-target species, and persist in the environment.
INTERFERENCE TESTS
For products containing two or more antigenic components, testing must establish that there is no interaction between the separate components, which would mean that one component wouldn’t boost the immune system’s defences against another.
CONSISTENCY OF PRODUCTION
Each establishment should generate in its facilities three consecutive manufacturing batches or serials of the finished products before marketing approval of any new product to assess the consistency of production.
STABILITY TESTS
The legitimacy of the expiry date that appears on the product package must be established through stability studies (based on an accepted potency test).
7. Lot release
BATCH/SERIAL RELEASE FOR DISTRIBUTION:
The manufacturer is required to test each batch or serial for potency, safety, and purity before it is released.
- Serial/batch purity testing
Testing is used to identify various pollutants and determine purity.
Master seeds, primary cells, MCSs (Master cell stock), substances of animal origin that have not been sterilised (such as foetal bovine serum, bovine albumin, or trypsin), and every batch of the finished product are tested for contamination before being released.
2. Serial/batch safety test
When local and systemic reactions to vaccination with the batch that will be distributed are consistent with those mentioned in the registration dossier and product documentation, batches are deemed satisfactory.
SAMPLING: Samples should be selected from each batch/serial of product. The selector should pick a representative sample.
LABELLING: Criteria for labelling vary according to different standards in different countries.
FIELD TESTS (SAFETY AND EFFICACY)
PERFORMANCE MONITORING
Guidelines for Vaccination Usage in Disease Prevention Programs
In some instances, infectious disease agents threaten the well-being of humans, animals, the food supply, and the economy. Still, producers lack the financial means or sufficient incentives to pay for immunization. Examples of such circumstances include endemic diseases in poor nations, zoonotic and food-borne illnesses that do not seriously afflict livestock and emerging or exotic illnesses with a low likelihood of spreading to the target area. Governments or other organizations frequently need to provide financial support for projects aimed at disease prevention and eradication. An illustration would be the present enthusiasm for PPR elimination. No matter how the cost is covered, a low price per vaccination unit is important.
What is the vaccine?
A biological remedy that increases resistance to a particular disease is vaccination. A substance resembling a disease-causing germ is regularly used in vaccinations; it is frequently made from a bacteria’s less-powerful or dead forms, its toxins, or one of its surface proteins.
What are the different types of vaccines?
There are mainly 4-types of vaccine
1. Inactivated vaccine
2. Live vaccine
3. Viral vaccine
4. Toxoid vaccine
What are the different steps involved in vaccine production?
The main steps involved in vaccine production are the following:
1. Selecting the strain for vaccine production
2. Growing the microorganisms
3. Isolation and purification of microorganisms
4. Inactivation of microorganisms
5. Formulation of vaccine
6. Quality control
7. Lot release
Define the formulation of the vaccine.
Salts, preservatives, stabilizers, and buffer components may be used in the production of vaccines. Whether added, stored, or used, these additions shouldn’t hurt the vaccine’s active ingredients. Antibiotics, phenoxyethanol, phenol, and thimerosal are some of the stabilizers and preservatives utilized. Aluminium sulphate, potassium aluminium sulphate, and aluminium hydroxide are frequently used adjuvants in vaccines.
What is the difference between differential and density gradient centrifugation?
Differential Centrifugation
This method, which involves varying speeds and timings, is used to separate cell organelles. The resulting pellet and supernatant are then treated to various speeds at various periods, the supernatant is then removed, and the procedure is repeated. Little particles are left in the supernatant when fractions separate at low rates. Depending on their size, shape, and density, cells, organelles, and macromolecules can be separated using the density gradient centrifugation technique.
Density gradient Centrifugation
Depending on their size, shape, and density, cells, organelles, and macromolecules can be separated using the density gradient centrifugation technique.
a. Rate zonal centrifugation-Using rate-zonal centrifugation, molecules are separated according to their size, shape, and density.
b. Isopycnic centrifugation, a method for classifying molecules according to density. (The definition of “isopycnic” is “equal density”).
What is serial/batch purity testing?
Serial/batch purity testing
Testing is used to identify various pollutants and determine purity.
Master seeds, primary cells, MCSs (Master cell stock), substances of animal origin that have not been sterilized (such as foetal bovine serum, bovine albumin, or trypsin), and every batch of the finished product are tested for contamination before being released.
What are the different criteria for the selection of strain in vaccine production?
The effectiveness of the resulting vaccination and any side effects have an impact on the choice of the seed, among other things.
– The bacterial strain or cell line should, if at all possible, come from a reputable culture collection with a provenance that has been established and documented.
– Conversely, if the chosen vaccination strain is an “in-house” clinical isolate, a full history of the strain, including information on its isolation, identification, and upkeep for product registration, will need to be compiled.
For more information, you can use this source as a reference:
https://www.britannica.com/science/vaccine
comment below if you like the blog Vaccines: Definition, Step by step process and Types of vaccine and if its helped you, it means a lot and will give us the energy to work with the same enthusiasm in future! This is it for today. See you in the Next article, Thank you!
You will also like :
The Innate and Adaptive Immune System
Download the Free Book of Plant Physiology by Taiz and Zieger 6th edition
[Download] Plant Systematics by Gurcharan Singh PDF Book 3rd edition
If you want important notes and updates about exams on your mobile then you can join SACHIN’SBIOLOGY on Instagram or Facebook and can directly talk to the founder of Sachin’s Biology and Author of biologywala.com Mr Sachin Chavan M.Sc. NET JRF (AIR 21) GATE, MH-SET