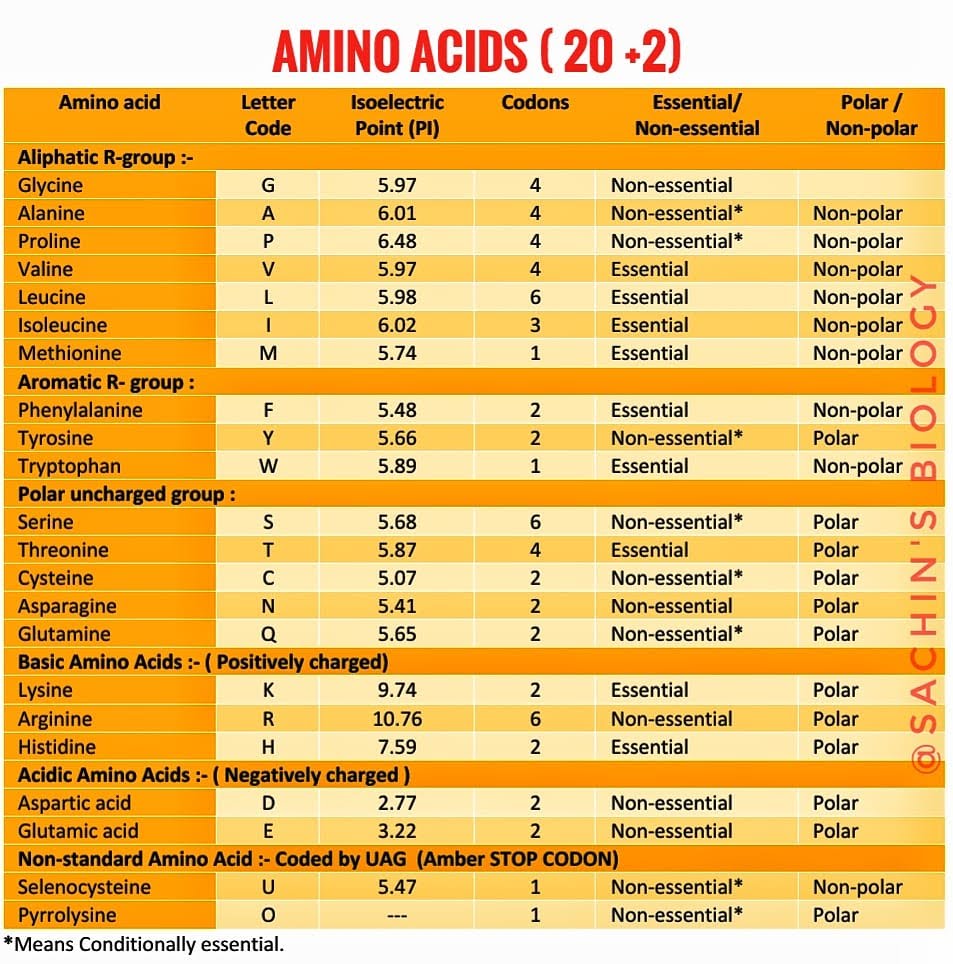
20 Standard Amino acids: The Best Possible Information

Watch this video before reading the notes to get all the basic information about Amiamino acid’s 20 types and their structures.
Classification & Important characters of 20 standard amino acids :
I. Non-polar aliphatic R-chain Amino Acids:
1)Glycine: (G)
Glycine is the smallest amino acid as it has a hydrogen atom has its side chain. Hence it has minimum steric hindrance which allows much more structural flexibility as compared to other standard amino acids in the protein.
This is the main reason why it occupies a maximum area on the Ramachandran plot. It has a Molecular weight of 75 daltons. Glycine is present in ample amounts in proteins like silk collagen (33%), fibroin (40%), and gelatin ( 25%).
Glycine doesn’t have chiral carbon hence it is the only standard amino acid that has only one stereoisomer and is optically inactive. It can be categorised into both hydrophilic or hydrophobic amino acids as it can fit into a hydrophilic or hydrophobic environment because of its minimal side chain of a single hydrogen atom.
Glycine is an inhibitory neurotransmitter in CNS. Glycine is a required co-agonist along with glutamate for NMDA receptors.
Glycine serves as a buffering agent, maintaining PH and preventing simple damage during electrophoresis hence it is used as a significant component of some solutions used in SDS PAGE.
Download the image containing a structure of amino acids 20 types from the link given at the end of this article.
2) Alanine: (A)
Alanine is a small non-essential standard amino acid for humans. it contains methyl group in its side chain which makes it non-polar aliphatic amino acid. It has a Molecular weight of 89 daltons.
it’s an important source of energy for muscles and the central nervous system which strengthens the immune system and helps in the metabolism of carbohydrates and displays cholesterol-reducing effects in animals.
In mammals, alanine plays a key role in a glucose-alanine cycle between tissue and liver. Alteration in the alanine cycle results in increasing levels of serum alanine aminotransferase (ALT) is linked to the development of type 2 diabetes. Generally, the L-form of amino acids are commonly found in nature here in the case of alanine, D-alanine also occurs in proteins of the bacterial cell wall and few antibodies.
II.Aliphatic branched chain amino acid:
3) Valine: (V)
Valine is an aliphatic and highly hydrophobic amino acid as compared to other aliphatic standard amino acids. Generally, it is found in the interior of globular proteins. All aliphatic branches and amino acids are essential amino acids. Any defect in the metabolism of branched-chain amino acids leads to genetic metabolic disorder, maple syrup urine disease. It’s due to non-functional branched chain alpha- ketoacid dehydrogenase.
Valine is a precursor in the penicillin biosynthetic pathway. It also plays important role in muscle growth and tissue repair. The deficiency of Valine results in neurological defects in the brain. It is primarily found in mitochondria and extracellular space.
4) Leucine: (L)
Leucine is an important essential, branch chain amino acid. Even it’s nonpolar it is sparingly soluble in water. The L form of leucine is primarily found in mitochondria or extracellular space. It can be found in bio-fluids like blood, cerebrospinal fluid (CSF) sweat, faeces and in most human tissues.
5) Isoleucine: (I)
Isoleucine is a branching essential amino acid. It is an isomer of leucine that has two chiral carbon atoms. Hence occurs in four stereoisomeric forms. Isoleucine can convert into both Acetyl CoA and malate during catabolism so that it’s both a glucogenic and ketogenic amino acid. Higher levels of isoleucine are observed in the blood of diabetic mammals, hence like other branched-chain standard amino acids, it is associated with insulin resistance.
6) Methionine: (M)
Methionine is a sulphur containing essential amino acids. In the process of protein synthesis, it is the first amino acid that gets translated into a polypeptide chain. It is an intermediate in transmethylation reaction. Where it serves as a major methyl group donor, even for DNA and RNA intermediates.
Methionine is the metabolic precursor for cysteine. In plants, Methionine is also used to synthesise ethylene. Cereals have an insufficient quantity of Methionine whereas pulses lack it.

II. Aromatic R chain Amino Acids:
Phenylalanine, tyrosine and tryptophan are aromatic amino acids. Phenylalanine and tryptophan are essential amino acids and they are produced by the shikimic acid pathway in plants and bacteria which is absent in humans. Tyrosine can be produced by phenylalanine.
Tryptophan, tyrosine and phenylalanine can absorb UV light Between 262- 280 nm with maxima at 280 nm. Due to their conjugated double bonds of all three amino acids can absorb UV light.
7) Phenylalanine: (F)
It is a nonpolar, essential amino acid. It absorbs maximum UV light at 260 nm wavelength due to the aromatic ring of benzene. It is a precursor for tyrosine and tyrosine derived metabolites. It crosses the blood-brain barrier by using the same active transport channel as tryptophan. It may interfere with the production of serotonin aromatic amino acids and nitric oxide, when present in excess quantity.
Phenylalanine is found naturally in the breast milk of mammals. Lack of enzyme phenylalanine hydroxylase main causes autosomal genetic disorder phenylketonuria ( PKU) which is the inability to metabolize phenylalanine. Excess of phenylalanine oxidized into phenyl pyruvic acid (phenyl ketone) which can cross blood-brain barrier and affects the functioning of the brain.
8) Tyrosine: (Y)
Tyrosine is a non-essential amino acid with hydroxylated benzene as a polar side group. Phenylalanine is act as a precursor for tyrosine by the activity of phenylalanine hydroxylase.
It can be used for phosphorylation by protein kinases for signal transduction purposes. Tyrosine act as a precursor for skin pigment like melanin. Tyrosine also acts as a precursor for the thyroid hormones like triiodothyronine (T3) and thyroxine (T4).
In the process of photosynthesis in chloroplast photosystem II, it acts as the electron donor to chlorophyll. The phenolic (-OH) group loses its hydrogen and reduces the photosystem II which further tyrosine gets reduced by the four core manganese cluster.
The plant-like Papaver somniferum convert tyrosine into alkaloid morphine.
9.Tryptophan: (W)
Tryptophan is an essential amino acid and shows maximum absorption of UV light at 280 nm. It is the most complex heterocyclic amino acid which is a derivative of indole.
Emission of fluorescence folded protein is due to excitation of tryptophan residue and lesser extent from tyrosine.
Tryptophan is the precursor of :
- Serotonin (neurotransmitter)
- Auxins (phytochromes)
- Niacin (vitamin B3)
- Melatonin (neurohormone).
III. Acidic Amino Acids:
10. Aspartic acid: (D)
Aspartic acid is a non-essential amino acid. Frequently synthesized from transamination reaction of oxaloacetate. Aspartate weakly stimulates NMDA receptors (The N-methyl-D-aspartate receptor is a glutamate receptor and ion channel protein found in nerve cells. The NMDA receptor is one of three types of ionotropic glutamate receptors. The other receptors are the AMPA and kainate receptors).
At pH 7.4 in proteins R– chain usually occurs as negatively charged aspartate -COO_, it bears a negative charge at pH 7, hence the isoelectric point is less than 7.
This amino acid is found commonly as D-aspartate in mammals. Aspartic acid plays an important role in its ionic form in the malate-aspartate shuttle. In the urea cycle formation of Urea takes place because of aspartate and ammonia donate amino group. While in the chain of ATP synthase it acts as hydrogen acceptor.
12. Glutamic acid: (E)
Glutamic acid is a non-essential amino acid that acts as an excitatory neurotransmitter in the vertebrates in its ionic form of glutamate. It can produce from the transamination of Alpha-ketoglutarate. It plays important role in the excretion of excess and waste nitrogen from the body. Oxidative deamination of glutamate catalyses by glutamate dehydrogenase.
For the umami test glutamic acid is often used as a food additive and flavour enhancer in the sodium salt form has MonoSodium Glutamate (MSG). Glutamate also served as the precursor for gamma-aminobutyric acid (GABA), a decrease in DABA synthesis is due to Stiff Person Syndrome. ( It is a neurological disorder caused by anti gad antibodies that results in a decrease in GABA synthesis, which causes damage to the pancreas which may lead to diabetes and impaired motor function like muscle stiffness and spasm.
IV. Basic Amino Acids:
12) Lysine: (K)
Lysine is a nutritionally important basic amino acid. Which is not synthesized by animals, but pulses contain a good amount of lysine where cereals lack in it. The amino group takes part as a base in catalysis.
Following are the post-translational modification that can be done on Lysine:
- Methylation
- Acetylation
- Sumoylation
- Ubiquitination
- Hydroxylation
Hydroxylysine is residues plays important role in all linked glycosylation in the endoplasmic reticulum and Golgi complex for secretion of proteins from the cell.
Lysine act as a conserved residue in opsins like rhodopsin and visual opsins. Deficiency of this may cause blindness and many more due to its ubiquitous presence in proteins.
13) Arginine: (R)
Arginine is classified as a semi-essential or conditionally essential amino acid as its requirement depends upon developmental stages and the health status of the particular individual in humans. found in highly basic proteins like protamines in Salmon and herring sperm. In protamines, it may found about 80% of the total amino acids.
Arginine is a more basic amino acid than lysine due to the unique position of the guanidinium group. Ionic charges are a result of the protonation of the guanidinium group.
Arginine is the precursor of nitric oxide (NO), which is an important signalling molecule that acts as a secondary messenger and an intracellular messenger regulating vasodilation and functions in the immune system reaction to infection. While it is also a precursor of ornithine and urea.
Arginine also takes part in the post-translational modifications by deamination of R- chains like methylation and citrullination
It plays important role in the following processes:
- Cell division
- Wound healing
- Removing ammonia
- Immune function
- Release of hormones
14) Histidine: (H)
Histidine is a weak basic amino acid, it contains a weakly basic imidazolium R group with a pKa value of 6.0. The imidazole ring in histidine is aromatic but doesn’t absorb 280 nm UV but does in lower UV range 220nm. It is the only amino acid that dissociates its proton at neutral pH.
This is the property that allows histidine to take part in acid-base catalysis. The imidazole side chain plays important role in coordination with ligand in metalloproteins and acts as catalytic sides in certain enzymes.
Imidazole chain is also responsible for phosphorylation by kinases during cell signalling in bacteria and plants as both have a two-component system and history is an integral part of a two-component system.
Histidine plays an important role in stabilizing oxyhaemoglobin and destabilizing CO bound haemoglobin, because of that carbon monoxide binding generally which is 20000 times stronger in free heme gets reduced in haemoglobin as only 200 times stronger.
Histidine acts as the precursor for histamine which is necessary for the inflammation process in the body.
V. Polar amino acids:
15) Serine: (S)
Silk protein, sericin and fibroin show a high portion of serine in their structures. The polarity of Serin is due to an alcoholic hydroxyl group. Which plays important role in the catalytic function of many enzymes ex. Serine protease.
Serine also takes part in O-linked glycosylation and its R-chain is generally phosphorylated by kinases for cell signalling in eukaryotes.
D-form of serine was initially found in bacteria and now in recent studies, it has been discovered that it naturally exists in humans too. D-form of serine act as a neuromodulator by activating the NMDA receptor, this D- form is synthesized in neurones by the L-form of serine.
16) Threonine: (T)
It has four stereoisomers because of two asymmetric carbon atoms present in a structure. L-threonine is the natural isomer and has an R-chain susceptible to many post-translational modifications.
The hydroxyl side chain can undergo O-linked glycosylation. While the serine-threonine kinase act on threonine residue to perform phosphorylation.
17) Cysteine: (C)
A single oxygen atom replaced by sulphur from Serine gives Cysteine molecule. When the same atom is replaced by selenium gives selenocysteine ( Important for CSIR NET JRF Exam). The Sulfhyhydryl group present in cysteine can easily be oxidized as it is nucleophilic.
It also possesses antioxidant properties hence it is the member of tripeptide (E-C-G) in antioxidant glutathione. It plays important role in anchoring iron in cytochrome p450 and nickel in [NiFe]-hydrogenase.
When two cysteine residues joined by disulfide bonds are called Cystine. The disulfide bonds play an important role in the folding and stability of protein, generally in secretory proteins present in the extracellular medium.
These bonds are usually unstable in cytosol except keratin. Disulfide bonds in the system also absorb UV light of wavelength 255 nm.
Cysteine also takes part in many post-translational modifications like prenylation. The sulfhydryl group act as an active site of enzymes like caspase and some cysteine protease like papain.
18) Asparagine: (N)
Asparagine is a non-essential neutral amino acid with an amide R-chain. The structure of Asparagine contains two buffering zones. Asparagine residue is generally found near the beginning of the Alpha helix as the side chain can form hydrogen bond interaction with the peptide backbone as ASX turns or ASX motifs.
When it comes to post-translational modification Asparagine provides key sides for N-linked glycosylation and modification of the protein by adding chains of carbohydrate.
19: Glutamine: (Q)
Glutamine is an important source of energy after glucose. Glutamine is the most abundant free amino acid present in human blood. It plays an important role in lipids synthesis and Protein synthesis, especially by cancer cells. It produces ammonium ions which helps in the acid-base balancing of the kidney.
It acts as a nitrogen donor for many anabolic processes like purine synthesis. It can form isopeptide linkages and can transfer non-toxic forms of ammonia in the blood circulation from the liver.
20.Proline: (P)
Proline is alpha imino acid as its Alpha-amino group has attached directly to the side chain. It can be found in both cis and trans isomeric forms.
In Alpha helix and beta sheets, proline act as structural disruptors when present in the middle of a regular secondary structure. It is also commonly found as the first residue of an Alpha helix and also in the edge stands of beta-sheets. Generally, it’s also found in secondary structures like beta-turn.
It may generate a polyproline helix by adding multiple proline and hydroxyproline in a row, which is a predominant structure in the collagen protein. The stability of collagen depends upon hydroxylation of proline by prolyl hydroxylase enzyme.
In ninhydrin, test proline produces an Orange/ yellow colour instead of blue/ purple colour like other standard amino acids. Proline also acts as osmolytes which help plants in osmoprotectant during a variety of stresses.
Unlike other standard amino acids, the phi and psi angles about peptide bond in proline have fewer allowable degrees of rotation, because of which proline occupies the least area on Ramachandran plot as compared to other standard amino acids.
20+1) Selenocysteine:
It is the 21st proteogenic amino acid, the highlighting point about selenocysteine is that it’s coded by stop codon UAG umber, which makes it different from standard amino acids. Selenocysteine is non-polar in nature and non-essential but in some developmental stages, it becomes essential Hence called conditionally essential. Selenocysteine is an integral part of the significant enzyme glutathione peroxidase.
20+2) Pyrrolysine:
It is also a conditionally essential amino acid that is non-polar. Pyrrolysine is also coded by stop codon number UAG. As with standard amino acids, pyrrolysine is not part of human proteins but is present in methanogenic archaebacteria.
If you want o know more about Amino acids then watch :
Amino Acids are precursors for proteins, important metabolic intermediates neurotransmitters and precursors for nitrogen-containing compounds, glucose, ketone bodies, etc.
Amino acids are organic compounds composed of amino (NH2) and acidic (COO-) functional groups.
Amino acids have both amino and carboxylic acid groups attached to Alpha carbon atom have particular importance. There are about 500 naturally occurring amino acids present in nature out of which 20 are standard amino acids commonly found in living organisms.
These 20 standard amino acids combine into a peptide chain to form the building blocks of proteins. All 20 standard amino acids show variation in R-chain attached to Alpha carbon.
Sterio-isomerism:
L-isomer is the most common form of Alpha-amino acid found naturally. Except for glycine Alpha carbon present in all standard amino acids is a chiral carbon atom ( attached with 4 different functional groups). Hence all Alpha-amino acids can exist in two enantiomers ‘L’ amino acid or ‘D’ amino acid which is nothing but mirror images of each other.
L-amino acids are the most common form in standard amino acids found naturally and in proteins during translation in the ribosome. While D-amino acids found some proteins produced by post-translational modifications after translation with the help of the endoplasmic reticulum.
‘D’ form of amino acids are not so common but found in peptidoglycan cell walls of bacteria. It is also found in other compounds like Tyrocidine and Valinomycin.
Zwitterion:
At pH range between 2.2 and 9.4, amino acid usually contains both a negative purpose highlight and positive Alpha ammonium group to form net Zero charge ion called Zwitterion.
The Alpha- a carboxylic acid group of amino acid is a weak acid and could be deprotonated to become negatively charged carboxylate ion ( _COO-). This negatively charged ion was found predominantly at a pH of less than 2.2. while on the other hand Alpha amine of amino acid is a weak base and could be protonated to become a positive Alpha ammonium group ( NH³+). This positively charged Alpha ammonium group predominates at pH values less than the pKa of the Alpha-ammonium group ( pH<9.4).
Isoelectric Point:
At the exact midpoint between two pKa values, the trace amount of net negative and trace amount of net positive ions exactly balance, show that the average net charge of all forms present is zero. This pH where charge your Biomolecule is zero is known as the isoelectric point ( pI).
Standard Amino Acids:
Naturally occurring proteins has 22 amino acids which are incorporated into polypeptides and are called proteinogenic or standard amino acids. Out of 22 amino acids, 20 are encoded by universal genetic code in the process of Central dogma while the remaining two named selenocysteine and pyrrolysine are incorporated into proteins by unique synthetic mechanisms.
Non standard amino acids:
Besides 22 standard amino acids, there are many non-standard amino acids also present naturally. Few amino acids do not become part of a protein called non-protein amino acids. These amino acids are found as intermediate in metabolic reactions for standard amino acids. Ex: Ornithine and Citrulline found in Urea Cycle. 2-aminobutyric acid and neurotransmitter Gamma-aminobutyric acid (GABA) are typical examples of non-standard amino acids.
Essential Amino Acids:
Simply these are amino acids that cannot be synthesized by our body, hence we need to take them from the diet. 9 Out of 20 standard amino acids are called essential amino acids which the human body cannot synthesize. These are Histidine, Isoleucine, Leucine, Lysine, Methionine, phenylalanine, Tryptophan, Valentine, Threonine.
Glucogenic and Ketogenic amino acids:
All standard amino acids are further divided into two categories glucogenic and ketogenic based on their stabilisation into malate or acetyl CoA respectively. This means the standard amino acids which can be catabolized only into Acetyl CoA can be converted into ketone bodies are termed ketogenic amino acids. While amino acids which can be catabolized into Malate, can be converted into glucose are termed glucogenic amino acids.
Only Ketogenic standard amino acids :
- Leusine
- Lysine
Only Glucogenic standard amino acids :
- Glycine
- Alanine
- Valine Proline
- Asparagine
- Aspartate
- Glutamate
- Glutamine
- Argenine
- Histidine
- Threonine
- Cysteine
- Methionine
Both glucogenic and ketogenic :
- Isoleucine
- Phenylalanine
- Tryptophan
- Tyrosine
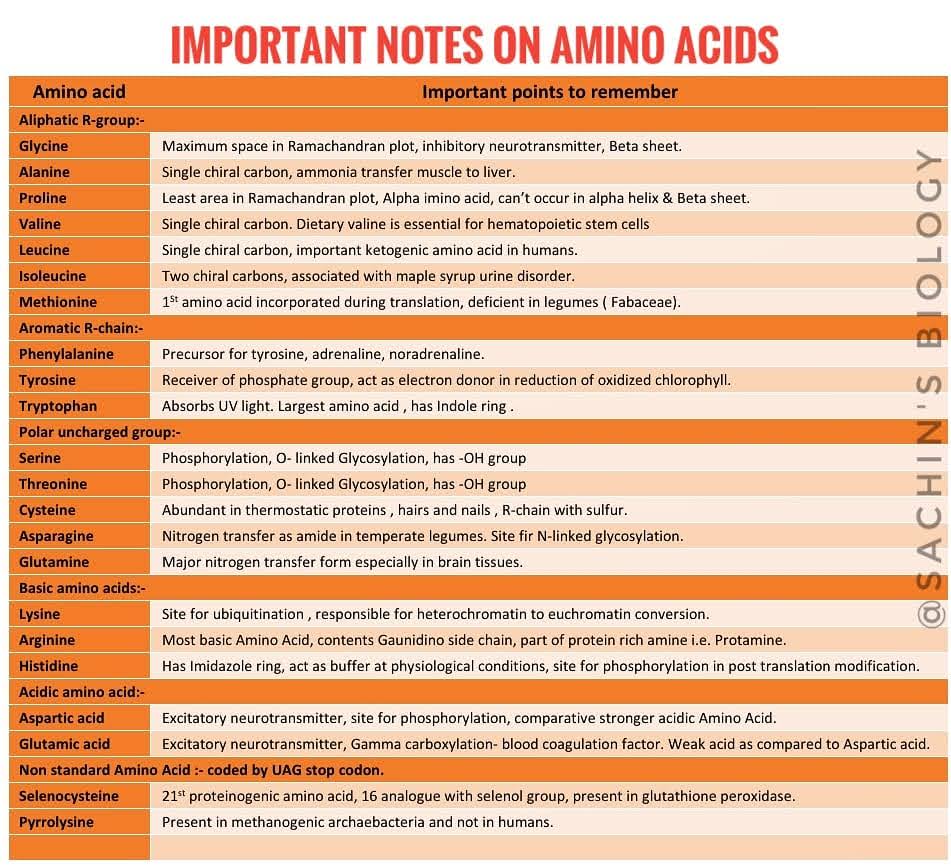
Important points for exam:
- Speciality in structure ( For DBT JRF Exam)
- Precursors of standard amino acids
- Post translation modifications
- Role in Physiological processes and in syndromes
- Special role in protein structures
- Biochemical properties like pKa and pI values.
This is it for Standard Amino Acids, you can download the image containing the structure of all standard amino acids from the link given below.
Download the Image of Structure of 20 Standard Amino Acids.
You will also like :
[FREE [Download] Campbell Biology Concepts & Connections 9th Edition pdf book
If you want important notes and updates about exams on your mobile then you can join SACHIN’SBIOLOGY on Instagram or Facebook and can directly talk to the founder of Sachin’s Biology and Author of biologywala.com Mr Sachin Chavan M.Sc. NET JRF (AIR 21) GATE!
What are 20 amino acids?

alanine – ala – A
arginine – arg – R
asparagine – asn
aspartic acid – asp – D
cysteine – cys – C
glutamine – gln – Q
glutamic acid – glu – E
glycine – gly – G
histidine – his – H
isoleucine – ile – I
leucine – leu – L
lysine – lys – K
methionine – met – M
phenylalanine – phe – F
proline – pro – P
serine – ser – S
threonine – thr – T
tryptophan – trp – W
tyrosine – tyr – Y
valine – val – V


![[Download] A Text Book Of Practical Botany 2 | Bendre Kumar Practical Botany PDF Book 8 [Download] A Text Book Of Practical Botany 2 | Bendre Kumar practical botany pdf book](https://biologywala.com/wp-content/uploads/2022/04/A-Text-Book-Of-Practical-Botany-2-FEATURED-520x245.webp)

perfect👍
Nothing is remaining…..all set about Amino acids ..👍😍
Awesome